Research Overview
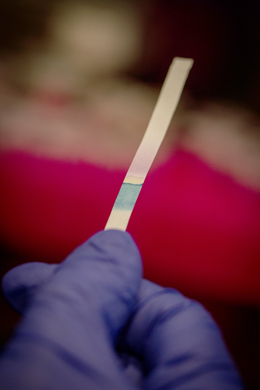
Environmental Analytes Test Strips
One of the first projects being undertaken by the Biointerfaces Institute is the development of test strips for detection of environmental analytes (e.g. pesticides, heavy metals and E. coli). Prototype strips have already been developed with good bioactivity and long-term stability. Portable bio-sensing papers are expected to be useful in monitoring environmental and food-based toxins, as well as in remote settings in less industrialized countries where simple bioassays are essential for the first stages of detecting disease.
This work is protected by a pending patent, and has been published in leading scientific journals, describing its application for agriculture and food safety. One of the major differences between these test strips and more common immunodiagnostic strips is the use of enzyme assays on paper. This format has been used for assaying enzyme inhibitors, substrates and cofactors, with extensive studies in both colorimetric and fluorometric enzyme assays.
Research at the Institute
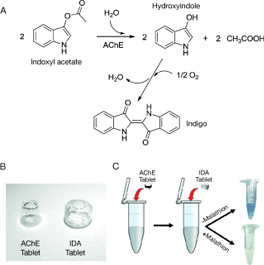
Pullulan Encapsulation of Labile Biomolecules
A simple and inexpensive method is reported for the long-term stabilization of enzymes and other unstable reagents in premeasured quantities in water-soluble tablets (cast, not compressed) made with pullulan, a nonionic polysaccharide that forms an oxygen impermeable solid upon drying. The pullulan tablets dissolve in aqueous solutions in seconds, thereby facilitating the easy execution of bioassays at remote sites with no need for special reagent handling and liquid pipetting. This approach is modular in nature, thus allowing the creation of individual tablets for enzymes and their substrates.
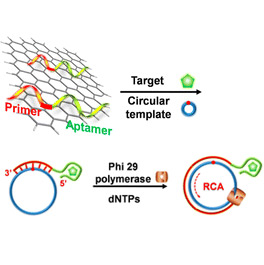
A Graphene-Based Biosensing Platform
We report a versatile biosensing platform capable of achieving ultrasensitive detection of both small-molecule and macromolecular targets. The system features three components: reduced graphene oxide for its ability to adsorb single-stranded DNA molecules nonspecifically, DNA aptamers for their ability to bind reduced graphene oxide but undergo target-induced conformational changes that facilitate their release from the reduced graphene oxide surface, and rolling circle amplification (RCA) for its ability to amplify a primer-template recognition event into repetitive sequence units that can be easily detected.
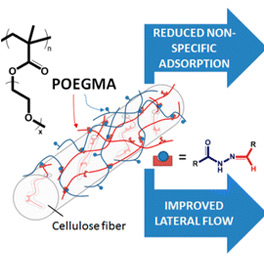
Turning Cellulose Paper into a Protein-Repellent Platform for Biosensors
The passivation of nonspecific protein adsorption to paper is a major barrier to the use of paper as a platform for microfluidic bioassays. Herein we describe a simple, scalable protocol based on adsorption and cross-linking of poly(oligoethylene glycol methacrylate) (POEGMA) derivatives that reduces nonspecific adsorption of a range of proteins to filter paper by at least 1 order of magnitude without significantly changing the fiber morphology or paper macroporosity.